How Many Valence Electrons In Chromium. The “octet rule” does not apply to the elements in the d and f blocks (the middle of the periodic table) because their outer electrons are not in the s and p subshells, but in the d (and/or f) subshells, and it takes more than 8 electrons to fill these. How many electrons are in an uncharged atom?
According to the periodic table above, phosphorus belongs to group 5a. Therefore, the valence electrons of cobalt(co) are nine. Its electron configuration as an atom is [ar]3d54s1, so it has 6 valence electrons.
The “Octet Rule” Does Not Apply To The Elements In The D And F Blocks (The Middle Of The Periodic Table) Because Their Outer Electrons Are Not In The S And P Subshells, But In The D (And/Or F) Subshells, And It Takes More Than 8 Electrons To Fill These.
(atomic number 23) vanadium core electrons vanadium orbital diagram vanadium valence electron configuration why does vanadium have 5 valence electrons It is classified as a transition metal. There are 6 valence electrons.
Chromium Lies In Group 6 Of The Periodic Table ;
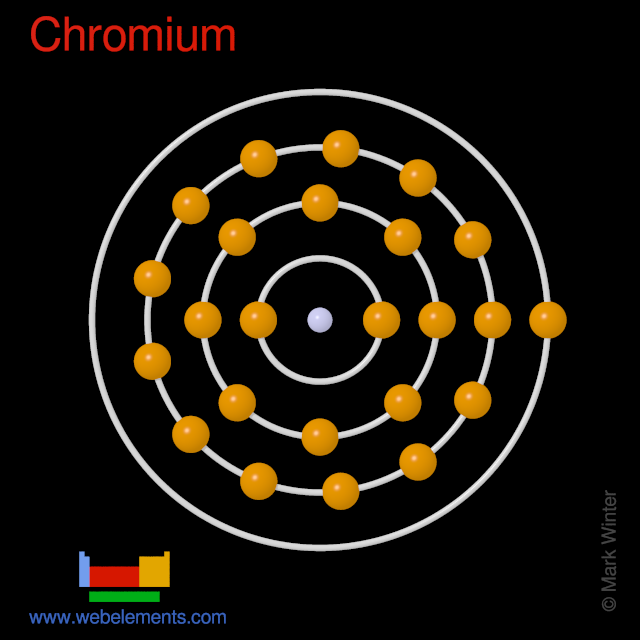
The electronic configuration of chromium is 3d5 4s1 i.e. How many electrons are in an atom of chromium? Chromium is the first element in the sixth column of the periodic table.
In Order To Write The Chromium Electron Configuration We First Need To Know The Number Of Electrons For The Cr Atom (There Are 24 Electrons).
In the case of chromium the valence electrons is 2,3,6. Chromium has six valence electrons. Valence electrons help chemical bonding.
If An Element Lacks A Valence Electron Or Is In The Octet State (Noble Gases), It Will Not Form Bonds Unless It Is Given Enough Energy To Break The Electrons Out Of The Octet State And Bond.
The valence electrons of chromium include its 4s and 3d electrons, because they are close enough in energy that more than one electron can be used to bond. Electrons have a negative charge. When we write the configuration we'll put all 24 electrons in orbitals around the nucleus of the chromium atom.
When We Write The Configuration We’ll Put All 19 Electrons In Orbitals Around The Nucleus Of The Potassium Atom.
How do you find the unpaired electrons in chromium? In writing the electron configuration for potassium the first two electrons will go in the 1s orbital. This is necessary because cr is a transition metal.