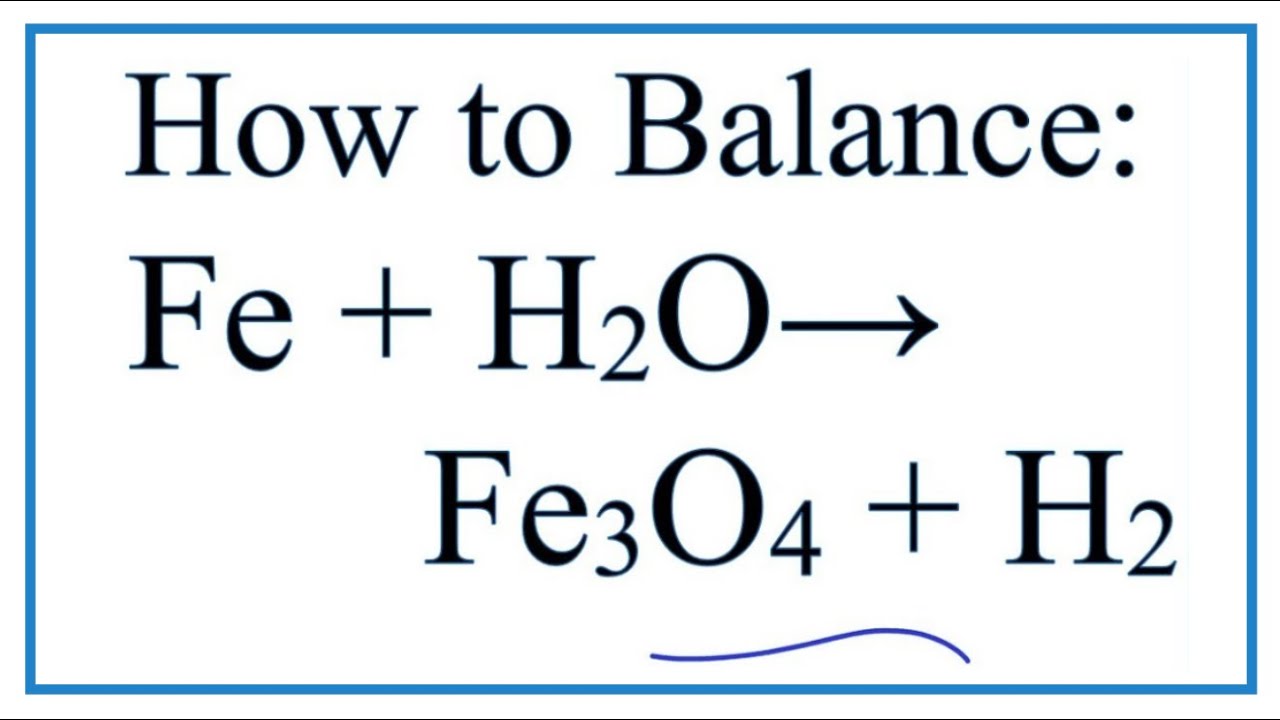Fe O2 Fe3O4. Fe + h2so4 → fe2(so4)3 + so2 + h2o; Fe + s → fes;
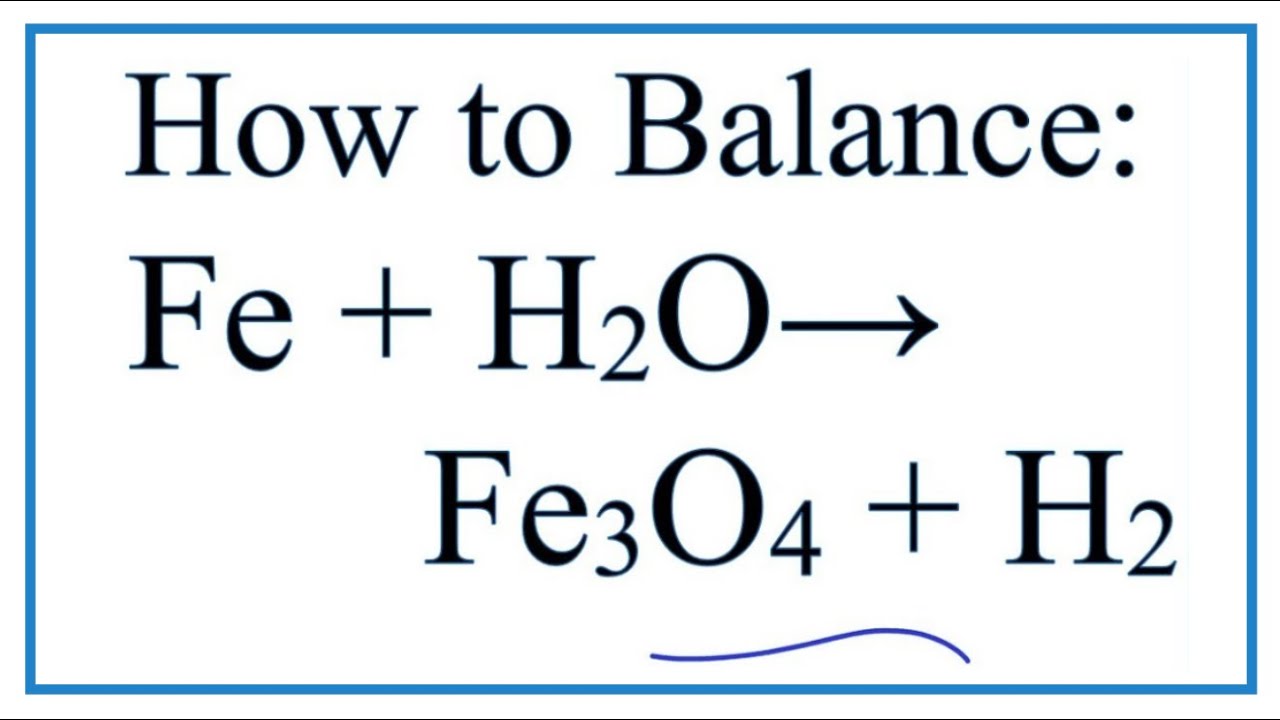 How to Balance Fe + H2O = Fe3O4 + H2 (Iron + H2O in the from www.youtube.com
How to Balance Fe + H2O = Fe3O4 + H2 (Iron + H2O in the from www.youtube.comLabel each compound with a variable a fe + b o 2 = c fe 3 o 4 2. Fe + hno3 → fe(no3)3 + n2o + h2o; La combustión de hierro en el aire.
K 4 Fe (Cn) 6 + H 2 So 4 + H 2 O = K 2 So 4 + Feso 4 + (Nh 4) 2 So 4 + Co.
Fe + s → fes; Fe + cl2 = fecl3; This iron oxide is encountered in the laboratory.
112 Parts Of Fe Powder Can Make Fe2O3 Transform Into Fe3O4 Completely.
Fe + s → fes; It occurs in nature as the mineral magnetite. 3fe + 4h 2 o → fe 3 o 4 + 4h 2
Find Another Reaction Thermodynamic Properties Of Substances
Examples of complete chemical equations to balance: 3fe + 2o 2 fe 3 o 4. Fe + o2 = fe3o4.
Balanced Chemical Reaction Equation With Reactants Fe (Iron) O2 (Oxygen) And Products Fe3O4 (Iron(Ii,Iii) Oxide) |.
Fecl2 + cl2 → fecl3 C2h4 o2 co2 h2o 3. 0a + 2 b = 4 c 3.
Màu Trắng Xám Của Sắt (Fe) Dần Chuyển Sang Màu Nâu Thành Hợp Chất Oxit Sắt Từ (Fe3O4).
Fecl2 + agno3 → fe(no3)2 + agcl; Or if any of the following reactant substances fe3o4 (iron(ii,iii) oxide), disappearing Al + o2 al2o3 3.