Sih4 Electron Dot Structure. B) which element can accommodate more than eight electrons in its valence shell? What is the lewis dot structure of sicl4?
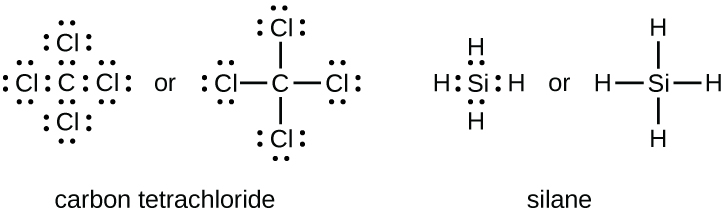
How many lone pairs of electrons exist on the central atom in ammonia? The molecular geometry of sih4 is tetrahedral with symmetric charge di. In the lewis structure of sf2, the central atom forms two bonds with two fluorine atoms and has two lone pairs of electrons.
When You Draw The Lewis Structure For Silicon You’ll Put Four “Dots” Or Valance Electrons Around The Element Symbol (Si).
Electron dot structure for outer atom: In a lewis dot structure, a lone pair of electrons are electrons that are unshared. Put the si in the center, hydrogens always go on the outside.
Electron Dot Structure For Outer Atom:
Let's do the lewis structure for sih4. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. How many lone pairs of electrons exist on the central atom in ammonia?
Sp3 Then Draw The 3D Molecular Structure Using Vsepr Rules:
(a) h2o(b) nh3(c) bh3(d) ch4(e) sih4 has a central atom with less than an octet of electrons In the lewis structure of sf2, the central atom forms two bonds with two fluorine atoms and has two lone pairs of electrons. This means that they have not bonded with any other atom.
It Is Even Lighter Than Air And Can Be A Cause Of Skin And Eye Irritation.
The two lone pairs of electrons push the fluorine atoms downwards due to the repulsive forces, and as a result, the shape of this molecule is bent. B) which element can accommodate more than eight electrons in its valence shell? Lewis dot structures are commonly referred to as electron dot structures or lewis structures.
Lewis Structures Are A Way To Show The Structure Of Molecules That Have.
Lewis structure sih4 bonding pairs lone pair wikipedia, chem 109a clas 1 1 0 2 6 2 2 8 5 3 1 8 4 4 0 8, what is the lewis dot structure for sih4 answers com, lewis structures or electron dot structures thoughtco, answered the total number of nonbonding electron pairs, hcn lewis structure w free video guide biochemhelp com, schupf computational It has a molar mass of 32.117 g/mol and a density of 1.313 g/l. Since it is in group 4 it will have 4 valence electrons.